Spectroscopy is a powerful analytical technique that involves the interaction of light with matter. At its core, spectroscopy is based on the principle that different substances absorb, emit, or scatter light in unique ways. This interaction can provide invaluable information about the composition, structure, and properties of materials.
The fundamental concept revolves around the electromagnetic spectrum, which encompasses a range of wavelengths from gamma rays to radio waves. Each type of electromagnetic radiation interacts with matter differently, leading to a variety of spectroscopic techniques. The science of spectroscopy hinges on the quantized nature of energy levels in atoms and molecules.
When light interacts with a sample, it can cause electrons to transition between these energy levels. The specific wavelengths of light absorbed or emitted during these transitions create a spectrum that serves as a fingerprint for the substance being analyzed. By studying these spectra, scientists can deduce critical information about molecular structures, chemical bonds, and even the physical state of a material.
This foundational understanding of spectroscopy sets the stage for its diverse applications across various scientific disciplines.
Key Takeaways
- Spectroscopy is the study of the interaction between matter and electromagnetic radiation, providing valuable information about the composition and structure of substances.
- There are various types of spectroscopy, including UV-Vis, infrared, and nuclear magnetic resonance spectroscopy, each utilizing different regions of the electromagnetic spectrum to analyze samples.
- Spectroscopy helps scientists unveil the composition and structure of matter by measuring the absorption, emission, or scattering of light, allowing for the identification of chemical compounds and the determination of molecular structures.
- Spectroscopy finds applications in a wide range of fields, from astronomy and environmental science to pharmaceuticals and forensics, enabling the analysis of celestial bodies, environmental pollutants, and biological samples.
- Advancements in spectroscopy technology and techniques, such as the development of miniaturized and portable spectrometers, have expanded its capabilities and made it more accessible for various applications.
The Different Types of Spectroscopy and How They Work
Spectroscopy encompasses a wide array of techniques, each tailored to specific types of analysis and materials. One of the most commonly used forms is infrared (IR) spectroscopy, which focuses on the absorption of infrared light by molecular vibrations. In IR spectroscopy, molecules absorb specific wavelengths corresponding to their vibrational modes, resulting in a spectrum that reveals functional groups and molecular structures.
This technique is particularly useful in organic chemistry for identifying compounds and assessing purity. Another significant type is nuclear magnetic resonance (NMR) spectroscopy, which exploits the magnetic properties of atomic nuclei. In NMR, samples are placed in a strong magnetic field and exposed to radiofrequency radiation.
The resulting interactions provide detailed information about the local environment of specific nuclei, allowing chemists to deduce molecular structures and dynamics. NMR is invaluable in fields such as drug development and biochemistry, where understanding molecular interactions is crucial. Additionally, mass spectrometry (MS) is often coupled with other spectroscopic techniques to provide complementary data.
While not a spectroscopic method in the traditional sense, MS analyzes the mass-to-charge ratio of ions generated from a sample. This information can be used to determine molecular weights and structural information about compounds. When combined with techniques like gas chromatography (GC) or liquid chromatography (LC), mass spectrometry becomes an essential tool for complex mixture analysis.
How Spectroscopy Helps Unveil the Composition and Structure of Matter
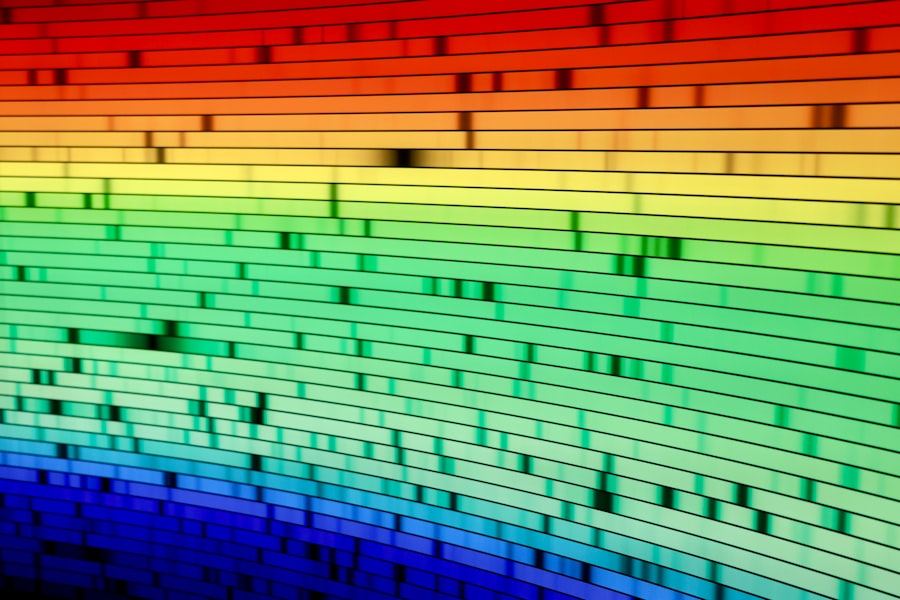
The ability of spectroscopy to reveal the composition and structure of matter is one of its most significant contributions to science. By analyzing the spectra produced during light-matter interactions, researchers can identify specific elements and compounds present in a sample. For instance, atomic absorption spectroscopy (AAS) allows for the detection of trace metals in environmental samples by measuring the absorption of light at characteristic wavelengths corresponding to those metals.
Moreover, spectroscopy can elucidate molecular structures by providing insights into bond types and arrangements. Techniques such as UV-Vis spectroscopy enable scientists to study electronic transitions within molecules, offering clues about conjugated systems and functional groups. The resulting spectra can indicate whether a compound is aromatic or aliphatic, saturated or unsaturated, thereby guiding chemists in their synthetic endeavors.
In addition to identifying individual components, spectroscopy can also be employed to analyze complex mixtures. For example, in food science, near-infrared (NIR) spectroscopy is used to assess the quality and composition of agricultural products without requiring extensive sample preparation. By examining the absorption patterns associated with various chemical constituents like carbohydrates, proteins, and fats, researchers can evaluate nutritional content and detect adulteration.
Applications of Spectroscopy in Various Fields, from Astronomy to Chemistry
| Field | Application |
|---|---|
| Astronomy | Studying the composition and temperature of stars and galaxies |
| Chemistry | Identifying chemical compounds and analyzing molecular structures |
| Environmental Science | Monitoring air and water quality through spectroscopic analysis |
| Pharmaceuticals | Quality control and analysis of drug compounds |
| Forensics | Identifying substances at crime scenes and analyzing evidence |
The applications of spectroscopy span numerous fields, showcasing its versatility as an analytical tool. In astronomy, for instance, spectroscopy plays a pivotal role in understanding celestial bodies. By analyzing the light emitted or absorbed by stars and galaxies, astronomers can determine their composition, temperature, density, and motion.
The Doppler effect observed in spectral lines allows scientists to measure the redshift or blueshift of distant objects, providing insights into the expansion of the universe. In chemistry and materials science, spectroscopy is indispensable for characterizing new compounds and materials. Researchers utilize techniques like Raman spectroscopy to study molecular vibrations and crystal structures in solid-state materials.
This information is crucial for developing new materials with tailored properties for applications in electronics, photonics, and nanotechnology.
Techniques such as fluorescence spectroscopy are employed to detect biomarkers associated with diseases like cancer.
By analyzing the fluorescence emitted by specific molecules within biological samples, clinicians can gain insights into disease progression and treatment efficacy.
Advancements in Spectroscopy Technology and Techniques
The field of spectroscopy has witnessed remarkable advancements over recent years, driven by technological innovations that enhance sensitivity, resolution, and versatility. One notable development is the advent of portable spectrometers that allow for on-site analysis in various environments. These compact devices enable rapid assessments in fields such as environmental monitoring and food safety without the need for extensive laboratory infrastructure.
Another significant advancement is the integration of machine learning algorithms with spectroscopic data analysis. By employing artificial intelligence techniques, researchers can process vast amounts of spectral data more efficiently than traditional methods allow. Machine learning models can identify patterns and correlations within complex datasets, leading to improved accuracy in material identification and characterization.
Moreover, advancements in laser technology have propelled techniques like laser-induced breakdown spectroscopy (LIBS) into new realms of application. LIBS utilizes high-energy laser pulses to ablate material from a sample’s surface, creating a plasma that emits light characteristic of its elemental composition. This technique has gained traction in fields such as planetary exploration and mining due to its ability to analyze solid samples rapidly and non-destructively.
The Role of Spectroscopy in Analyzing Environmental and Biological Samples

Spectroscopy plays a crucial role in environmental science by providing tools for monitoring pollutants and assessing ecosystem health. Techniques such as UV-Vis spectroscopy are employed to measure concentrations of contaminants in water bodies by analyzing absorbance at specific wavelengths associated with pollutants like heavy metals or organic compounds. This capability is vital for ensuring compliance with environmental regulations and safeguarding public health.
In biological research, spectroscopy has become an indispensable tool for studying biomolecules and cellular processes. For example, fluorescence resonance energy transfer (FRET) is a technique that allows researchers to investigate interactions between proteins at the molecular level. By labeling proteins with fluorescent dyes and measuring energy transfer between them, scientists can gain insights into protein-protein interactions and cellular signaling pathways.
Additionally, mass spectrometry has revolutionized proteomics—the large-scale study of proteins—by enabling the identification and quantification of thousands of proteins within complex biological samples. Coupled with liquid chromatography (LC-MS), this approach allows researchers to analyze protein expression profiles under different conditions, providing valuable insights into disease mechanisms and potential therapeutic targets.
Challenges and Limitations in Spectroscopy and How Scientists Overcome Them
Despite its many advantages, spectroscopy is not without challenges and limitations that researchers must navigate. One significant issue is spectral overlap, where multiple components within a mixture produce similar spectral features that complicate analysis. This overlap can lead to difficulties in accurately quantifying individual components or identifying specific substances within complex samples.
To address these challenges, scientists often employ advanced data analysis techniques such as multivariate analysis or chemometrics. These methods allow researchers to extract meaningful information from complex datasets by identifying patterns that may not be apparent through traditional analysis alone. By leveraging statistical approaches, scientists can improve their ability to deconvolute overlapping spectra and enhance the reliability of their results.
Another limitation lies in sample preparation requirements for certain spectroscopic techniques.
Researchers are continually exploring innovative sample preparation methods that minimize these requirements while maintaining analytical integrity.
The Future of Spectroscopy: Potential Breakthroughs and Discoveries
Looking ahead, the future of spectroscopy holds immense promise for scientific discovery across various domains. One area poised for significant advancement is the development of hyperspectral imaging techniques that capture detailed spectral information across a wide range of wavelengths simultaneously. This technology has applications in agriculture for monitoring crop health and detecting diseases early on.
Furthermore, advancements in quantum technologies may lead to breakthroughs in spectroscopic sensitivity and resolution. Quantum-enhanced spectroscopic methods could enable researchers to detect trace amounts of substances with unprecedented accuracy, opening new avenues for environmental monitoring and medical diagnostics. As interdisciplinary collaborations continue to flourish between fields such as chemistry, biology, physics, and engineering, we can expect innovative applications of spectroscopy that transcend traditional boundaries.
The integration of spectroscopy with emerging technologies like nanotechnology and biotechnology will likely yield novel insights into complex systems and drive forward our understanding of matter at both macroscopic and microscopic levels. In summary, spectroscopy remains an essential tool across diverse scientific disciplines due to its ability to provide detailed information about matter’s composition and structure. As technology continues to evolve, so too will the capabilities of spectroscopic techniques—promising exciting discoveries that will shape our understanding of the natural world for years to come.
Spectroscopy, a powerful analytical technique used to study the interaction between matter and electromagnetic radiation, has profound implications in various scientific fields, including quantum mechanics. An interesting related article is “Introduction to Absolutism, Relativism, and Quantum Mechanics: Exploring Relativism in Logic,” which delves into the foundational principles of quantum mechanics, a field where spectroscopy plays a crucial role in understanding atomic and molecular structures. You can read more about it by visiting the article here.


















+ There are no comments
Add yours